Protocols
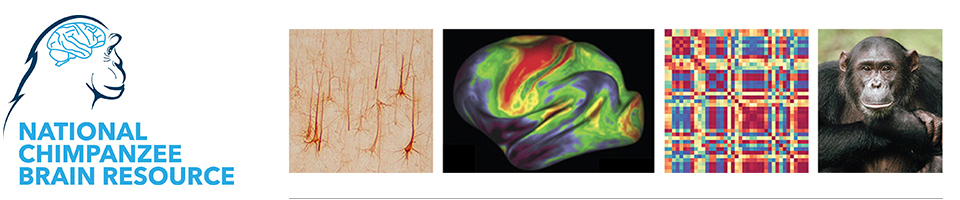
chimpanzee brain MRI overlaid on
skull. Image courtesy of Dr. Aida
Gómez-Robles.
All neuroimaging data and postmortem samples were collected according to accepted standards of animal care and with the approval of the relevant Institutional Animal Care and Use Committees (IACUC).
Magnetic Resonance Imaging
We have developed protocols for collecting high-resolution structural (T2) and diffusion-tensor imaging (DTI) scans in chimpanzees. The NCBR has both in vivo and postmortem formalin-fixed brain MRI data.
In vivo MRI procedure - Subjects were first immobilized with a telazol injection (2-6 mg/kg), and then anesthetized with propofol (10 mg/kg/h). For most MRIs, a 3 T scanner (Siemens Trio; Siemens Medical Solutions Inc., Malvern, PA, USA) was used to acquire structural scans.
In vivo DTI procedure - Diffusion-weighting gradients were applied in 60 directions with a b value of 1000 s/mm2, repetition time/echo time (TR/TE) of 5900/86 ms, field of view (FOV) of 130 × 230 mm2, matrix size of 72 × 128, partial Fourier option of 5/8, spatial resolution of 1.8 × 1.8 × 1.8 mm3, 41 contiguous slices covering the whole brain. Margosian reconstruction (Margosian et al., 1896) was used to overcome the phase distortions in diffusion-weighted partial Fourier acquisitions. Diffusion-weighted images with phase-encoding directions (left–right) of opposite polarity were acquired to correct for susceptibility distortion (Andersson et al., 2003). Eight averages of dw-MRI were collected to boost the signal noise ratio (SNR). For each average of diffusion-weighted images, 5 images without diffusion weighting (b = 0 s/mm2) were also acquired with matching imaging parameters. The total diffusion MRI scan time was approximately 60 minutes.
Post-mortem DTI procedure - Formalin-fixed brains were coated in a gadolinium-agar gel that solidifies without bubbles, reduces surface artifacts, and minimizes spatial distortion. DTI scans at 1.1 mm isotropic resolution were collected with a 3D segmented EPI sequence with 4 segments, 3 shells, and 128 directions, and 10 averages. To correct residual geometric distortion in the DTI data, we collected T2 structural images using a RARE sequence at the same spatial resolution.
Fixed Brains and Histological Sections
The NCBR collection includes intact fixed specimens, as well as histologically-prepared sections from chimpanzee brains. Brain tissue is collected postmortem from animals that have either died of natural causes or been euthanized for medical reasons. Upon collection from donating institutions, all brains are weighed and photographed from all angles and 3D surface models are created using photogrammetry techniques.
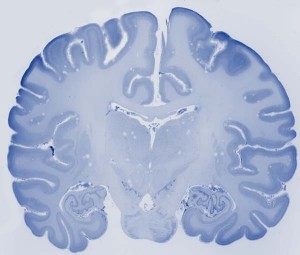
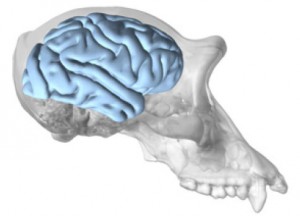
brain stained with cresyl violet.
Histological sectioning - In preparing each brain for histology, the hindbrain is separated from the cerebral hemisphere by sectioning at the level of the substantia nigra. The cerebral hemispheres are then bisected at the corpus callosum. Each whole hemisphere is cut into three coronal slabs, with one cut rostral to the precentral gyrus and one cut just rostral to the lunate sulcus. Based on our experience working with frozen sectioning, this blocking strategy reduces freezing artifact from temperature unevenness. Sections are cut at 40 µm thick using a freezing sliding microtome. Series of 1:10 sections are stained with cresyl violet to visualize cytoarchitecture and a 1:20 series is stained for myelin with the Gallyas method.
Tissue sections not used immediately in staining experiments are kept in anatomical order in separate numbered Eppendorf tubes and archived in a freezer storage solution consisting of glycerol, ethylene glycol, dH2O, and phosphate buffer (3:3:3:1 volume/volume) at -20°C to preserve antigen availability for future experiments. These stored sections are available from the NCBR upon request.
Frozen Brains
The protocol for collecting frozen brain samples is followed when tissue can be collected within 12 hours of death. Brains are cut in half along the midsagittal plane. The left hemisphere is cut into 2-cm blocks. Each block is placed in a freezer bag, numbered in order, and flash frozen in one of three ways: isopentane/dry ice at -30 to -40oC, liquid nitrogen, or -80oC freezer. Frozen sections are stored in a -80oC freezer. The right hemisphere is placed in 10% formalin solution.
Upon request, frozen samples will be dissected out, placed in individual tubes/bags and shipped overnight on dry ice. Dissections of tissue samples are collected for the user while 2-cm blocks remain frozen on slabs of dry ice. Between collections, all instruments, including the ice and gloves, are sanitized using RNA-Zap to prevent cross-contamination between individuals.